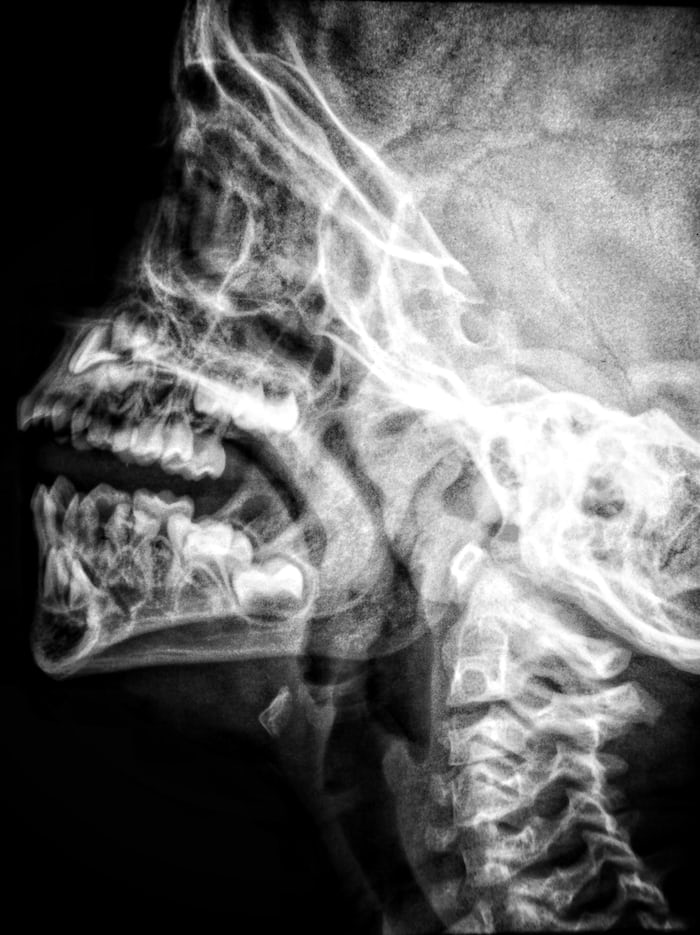
X-ray
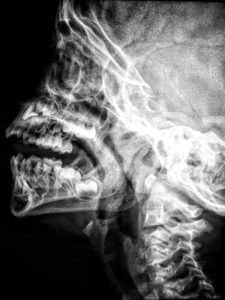
X-rays are electromagnetic waves with great energy and a very short wavelength that can pass through a variety of objects that are opaque (cannot be seen through under radiation) to light.
They are produced by an x-ray tube, which uses a high voltage to accelerate the electrons created by its cathode. X-rays are created when the generated electrons interact with the anode. Bremsstrahlung and the distinctive radiation of the anode element are among the x-rays generated.
X-rays can interact with matter by the following:
- The Photoelectric Effect
- Photodisintegration
- Compton Scattering
- Coherent, Rayleigh or Classical Scattering
- Pair production (not possible in the diagnostic radiology range)
Terminology in X-rays
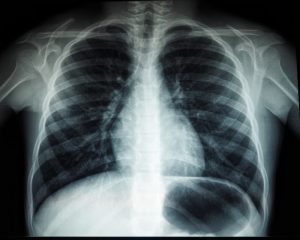
In addition to the electromagnetic radiation itself, the term “x-ray” is also used to describe the image produced, such as a chest x-ray. However, many radiologists, particularly in a more formal context, will claim that the term “radiograph” is the proper one for this.
History and Etymology of X-rays
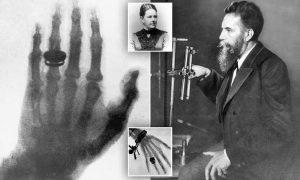
Wilhelm Roentgen (1845–1923), a German physicist, made the discovery of X-rays in 1895. Because Rontgen did not know what they were and was using the sign “x” to denote an uncertain number or object, they were called x-rays or x-radiation. He received the Physics Nobel Prize for his ground-breaking finding.
Properties of X-Rays
- Electromagnetic waves make up X-rays.
- X-rays have a wavelength that ranges from 1 to 100.
- In contrast to visible light, Ultraviolet, infrared, microwave, and radio waves, X-rays have high-energy waves.
- Ionization of matter’s molecules and atoms is a property of X-rays.
- They are capable of traveling at the speed of light, or 3 x 10^8 ms-1.
- X-rays have no electrical charge. Because of this, these beams are not deflected by magnetic or electric forces.
- In some metals, an X-ray can cause fluorescence and a photoelectric effect.
Application of X-rays
Electromagnetic radiation makes up X-rays. They are renowned for having the capacity to see through a person’s skin. They aid in displaying the images of the bones.
We can create more concentrated and potent X-rays with the aid of cutting-edge technologies. The imaging of tiny biological cells, the destruction of cancer cells, and the structural analysis of materials like cement are just a few uses for this potent and focused X-ray.
We also have numerous additional applications besides just employing X-rays for medical imaging.
- This can help with diagnosis. X-rays allow us to see the inside structures of bones and also aid in the early detection of bone fractures in people.
- Hard X-rays are useful in reducing the absorption of low-energy X-rays. A filter is placed on top of the X-ray tube to block transmission of the lower energy.
- Teeth and bones contain calcium atoms with a high atomic mass. This aids in X-ray absorption while allowing the majority of other radiation to flow through the body.
- X-ray diagnostic methods are also used in radiation, fluoroscopy, and CT scans (computer tomography).
- They are also helpful for cancer treatments with therapeutic procedures, as well as for fluorescence, spectroscopy, industrial radiography, crystallography, astronomy, microscopy, and imploding fission devices.
- This is useful in the creative industry as well. It is beneficial to both produce art and examine paintings.
Frequently Asked Questions (FAQs)
What type of radiation is an X-ray?
Ionizing radiation
X-ray machines pass x-ray beams (a form of ionizing radiation) through a part of the body to produce images of the tissue, organs, bones, or teeth inside.
What is the other name for X-ray?
In many languages, X-ray radiation is referred to as “Röntgen radiation,” after the German scientist Wilhelm Conrad Röntgen, who discovered it on November 8, 1895.
Which color has the most energy?
Violet
When it comes to visible light, the highest frequency color, which is violet, also has the most energy. The lowest frequency of visible light, which is red, has the least energy.
Interested in learning about other topics? Leave a comment below.